
LEVETIRACETAM, etiracetam
(-)-(S)-α-ethyl-2-oxo-1-pyrrolidine acetamide
(−)-(S)-alpha-ethyl-2-oxo-1-pyrrolidineacetamide
CAS…102767-28-2
Crystals from ethyl acetate, mp 117°. [a]25D -90.0° (c = 1 in acetone). Soly (g/100 ml): water 104.0; chloroform 65.3; methanol 53.6; ethanol 16.5; acetonitrile 5.7. Practically insol in n-hexane. LD50 in male mice, male rats (mg/kg): 1081, 1038 i.v. (Gobert, 1990).
Mp: mp 117°C
| Active Ingredient: | LEVETIRACETAM |
| Dosage Form;Route: | INJECTABLE;IV (INFUSION) |
| Proprietary Name: | KEPPRA |
| Applicant: | UCB INC |
| Strength: | 500MG/5ML (100MG/ML) |
| Application Number: | N021872 |
| Product Number: | 001 |
| Approval Date: | Jul 31, 2006 |
| Reference Listed Drug | Yes |
| RX/OTC/DISCN: | RX |
Levetiracetam is an anticonvulsant medication used to treat epilepsy. Levetiracetam may selectively prevent hypersynchronization of epileptiform burst firing and propagation of seizure activity. Levetiracetam binds to the synaptic vesicle protein SV2A, which is thought to be involved in the regulation of vesicle exocytosis. Although the molecular significance of levetiracetam binding to synaptic vesicle protein SV2A is not understood, levetiracetam and related analogs showed a rank order of affinity for SV2A which correlated with the potency of their antiseizure activity in audiogenic seizure-prone mice.
Epilepsy is a chronic neurological disorder that consists of repeated occurrences of spontaneous seizures. Levetiracetam, [(S)-a-ethyl-2-oxopyrrolidine acetamide], has recently been approved as an add-on therapy for the treatment of refractory epilepsy . The (S)-enantiomer of etiracetam (levetiracetam) has shown remarkable pharmacokinetic and pharmacological activity which has led to the quick approval of this antiepileptic drug by the FDA.
Levetiracetam offers several advantages over traditional therapy, including twice-daily dosing, a wide margin of safety with no requirements for serum drug concentration monitoring and no interactions with other anticonvulsants, and less adverse effects than traditional treatments
Levetiracetam (INN) /lɛvɨtɪˈræsɨtæm/ is an anticonvulsant medication used to treatepilepsy. It is the S-enantiomer of etiracetam, structurally similar to the prototypicalnootropic drug piracetam.
Levetiracetam is marketed under the trade name Keppra. Keppra is manufactured by UCB Pharmaceuticals Inc. Since November 2008 the drug has been available as a genericbrand in the United States.
Levetiracetam has been approved in the European Union as a monotherapy treatment for epilepsy in the case of partial seizures, or as an adjunctive therapy for partial, myoclonicand tonic-clonic seizures. It is also used in veterinary medicine for similar purposes.
Levetiracetam has potential benefits for other psychiatric and neurologic conditions such as Tourette syndrome, autism, bipolar disorder and anxiety disorder, as well asAlzheimer’s disease. However, its most serious adverse effects are behavioral, and its benefit-risk ratio in these conditions is not well understood.
Along with other anticonvulsants like gabapentin, it is also sometimes used to treatneuropathic pain. It has not been found to be useful for essential tremors.
Levetiracetam (LEV) is a novel antiepileptic drug (AED) which was discovered in early 1980s and soon, in 1999 FDA approved LEV for the management of partial onset seizure. In India, LEV tablet was approved in April 2005. It acts by binding to the synaptic vesicle protein SV2A, which is present on synaptic vesicles and some neuroendocrine cells. Pharmacokinetics of LEV such as, less protein binding and lack of hepatic metabolism makes LEV less susceptible to drug interactions with other anticonvulsants. Evidence also suggests that LEV is much better than other AEDs in the way of broad therapeutic window, convenient dosing and less adverse effect. Besides the pharmacological effects, pharmacoeconomically also, LEV is a beneficial drug. All these valuable pharmacological and pharmacoeconomic aspect makes LEV an important option in management of various types of epilepsy.

- PubMed Health A division of the National Library of Medicine at the National Institutes of Health.
- Keppra (levetiracetam) Final Printed Label April 2009. Center for Drug Evaluation and Research, U.S. Food and Drug Administration. Accessed 29 July 2011.
- Keppra UCB (manufacturer’s website)
- NIH MedLine drug information
KEPPRA injection is an antiepileptic drug available as a clear, colorless, sterile solution (100 mg/mL) for intravenous administration.
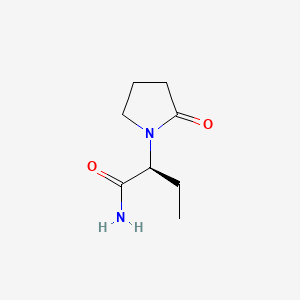
The chemical name of levetiracetam, a single enantiomer, is (-)-(S)-α-ethyl-2-oxo-1-pyrrolidine acetamide, its molecular formula is C8H14N2O2 and its molecular weight is 170.21. Levetiracetam is chemically unrelated to existing antiepileptic drugs (AEDs). It has the following structural formula:
 |
Levetiracetam is a white to off-white crystalline powder with a faint odor and a bitter taste. It is very soluble in water (104.0 g/100 mL). It is freely soluble inchloroform (65.3g/100 mL) and in methanol (53.6 g/100 mL), soluble in ethanol (16.5 g/100 mL), sparingly soluble in acetonitrile (5.7 g/100 mL) and practically insoluble in n-hexane. (Solubility limits are expressed as g/100 mL solvent.)
KEPPRA injection contains 100 mg of levetiracetam per mL. It is supplied in single-use 5 mL vials containing 500 mg levetiracetam, water for injection, 45 mg sodium chloride, and buffered at approximately pH 5.5 with glacial acetic acid and 8.2 mg sodium acetate trihydrate. KEPPRA injection must be diluted prior to intravenous infusion
(S)-(−)-α-ethyl-2-oxo-1-pyrrolidine acetamide, which is referred under the International Nonproprietary Name of Levetiracetam, its dextrorotatory enantiomer and related compounds. Levetiracetam is shown as having the following structure:
Levetiracetam, a laevorotary compound is disclosed as a protective agent for the treatment and the prevention of hypoxic and ischemic type aggressions of the central nervous system in the European patent No. 162036. This compound is also effective in the treatment of epilepsy, a therapeutic indication for which it has been demonstrated that its dextrorotatory enantiomer (R)-(+)-α-ethyl-2-oxo-1-pyrrolidine acetamide completely lacks activity (A. J. GOWER et al., Eur. J. Pharmacol., 222, (1992), 193-203). Finally, in the European patent application No. 0 645 139 this compound has been disclosed for its anxiolytic activity.
The asymmetric carbon atom carries a hydrogen atom (not shown) positioned above the plane of the paper. The preparation of Levetiracetam has been described in the European patent No. 0162 036 and in the British patent No. 2 225 322, both of which are assigned to the assignee of the present invention. The preparation of the dextrorotatory enantiomer (R)-(+)-α-ethyl-2-oxo-1-pyrrolidine acetamide has been described in the European patent No. 0165 919.
-
Several processes for obtaining levetiracetam have been disclosed. One promising approach is the reaction of (S)-2-aminobutyramide (5) with an alkyl 4-halobutyrate or with a 4-halobutyryl halide followed by cyclization as outlined in EP 162036 . Clearly, said (S)-2-aminobutyramide (5) is a key intermediate in the preparation of levetiracetam and given the importance of the correct stereochemistry of levetiracetam also the correct stereochemistry in the key intermediates is of importance.
-
The separation of stereoisomers is considered to be one of the difficult tasks in chemistry since chiral compounds exhibit identical physical properties in non-chiral environments. Although several approaches for the preparation of optically pure (S)-2-aminobutyramide (5) have been reported, many of these are related to resolution of racemic (R,S)-2-aminobutyramide (e.g. WO 2006/103696 ), optionally using catalytic amounts of an aldehyde such as described in JP 2007/191470 . However, an approach directly starting from the Schiff base of racemic (R,S)-2-aminobutyramide (i.e. compound (1)) is unavailable whereas there is a need for this as said Schiff bases are highly suitable from a preparative point of view as these compounds may be conveniently isolated from the aqueous media that they are usually prepared in. This is in contrast with the parent 2-aminobutyramide which is highly soluble in water and consequently difficult to obtain in sufficient purity.
British Pat. No. 1,309,692 describes the compound α-ethyl-2-oxo-l- pyrrolidineactamide (melting point 122 degrees C.) and states that compounds of this type can be used for therapeutic purposes, for example for the treatment of motion sickness, hyperkinesia, hypertonia and epilepsy.
-
The same document discloses obtaining levetiracetam by reacting (S)-2-aminobutanamide with an alkyl 4-halobutyrate or with a 4-halobutyryl halide, and subsequent cyclization of alkyl (S)-4-[[1-(aminocarbonyl)propyl]amino]butyrate or of (S)-N-[1-(aminocarbonyl)propyl]-4-halobutanamide thus obtained, as summarized in the attached scheme:
-
The two previous processes have the drawback of operating at temperatures between -10°C and -60°C and the drawback of using intermediates for cyclization that are not readily obtained.
-
A drawback of this industrial-scale process is that it requires special equipment and special precautions for handling the products.
-
Other processes are known (for example US patents No 6,107,492 and6,124,473 ) in which levetiracetam is obtained by means of optical resolution of racemic etiracetam of formula (I). InUS patent No 6,107,492 resolution is performed by means of preparative high performance liquid chromatography or by means of a continuous simulated fluid bed chromatographic system with a chiral stationary phase. US patent No 6,124,473 discloses a continuous simulated fluid bed chromatographic system consisting of at least three chiral stationary phase columns. These industrial-scale resolution processes are affected by drawbacks related to using chromatography.
-
The industrial-scale difficulties and hazard of hydrogenation can be mentioned in relation to these processes.
-
Finally, patent application ES 447,346 describes a process for the preparation of a pyrrolidone derivative, in particular the 2-oxo-1-pyrrolidinylacetamide, which comprises first reacting pyrrolidone with formaldehyde and a secondary amine, then reacting the compound obtained with an alkylating agent such as dimethyl sulfate, followed by treating the compound obtained with sodium or potassium cyanide, and finally reacting the compound obtained with hydrogen peroxide in basic medium.
Moreover, it also mentions that these compounds can be applied in the field of memory disorders in normal or pathological conditions.
It is also known that α-ethyl-2-oxo-l-pyrrolidineacetamide possesses a protective activity against aggressions of the central nervous system caused by hypoxias, cerebral ischemia, etc. (Pharmazie, 37/11, (1982), 753-765).
U.S. patent 4,969,943 discloses the levorotatory isomer of α-ethyl-2-oxo-l- pyrrolidineacetamide, which has the absolute S configuration, a method for making the isomer and pharmaceutical compositions containing the same. U.S. patent 4,696,943 discloses that the levorotatory isomer has a 10 times higher protective activity against hypoxia and a 4 times higher protective activity against ischemia compared to the known racemic form.

 http://oasys2.confex.com/acs/229nm/techprogram/P831117.HTM
http://oasys2.confex.com/acs/229nm/techprogram/P831117.HTM
Parallel synthesis of Levetiracetam (Keppra®) and its analogs via an Ugi-RCM strategy
…………………………

http://www.google.com/patents/US7939676
Levetiracetam, (−)-(S)-alpha-ethyl-2-oxo-1-pyrrolidineacetamide, is a drug useful as a protective agent for treating and preventing hypoxic and ischemic type aggressions of the central nervous system. It is the active ingredient of KEPPRA®, tablets and flavored liquid, indicated as adjunctive therapy in the treatment of partial onset seizures in adults and children four years of age and older with epilepsy.
Levetiracetam was first described in U.S. Pat. No. 4,837,223 (UCB Societe Anonyme) where it is stated that it has particular therapeutic properties compared to the known racemic form (non proprietary name etiracetam). The S-enantiomer, for example, has a ten times higher protective activity against hypoxia and a four times higher protective activity against cerebral ischemia than the racemic mixture.
The U.S. Pat. No. 4,837,223 describes a method for the preparation of levetiracetam which comprises reacting (−)-(S)-alpha-ethyl-2-oxo-1-pyrrolidineacetic acid successively with alkylhaloformate and with ammonia. Said acid intermediate is, in turn, obtained from racemic (±)-alpha-ethyl-2-oxo-1-pyrrolidine acetic acid by a classic optical resolution according to known methods. In example 1, ethyl (±)-alpha-ethyl-2-oxo-1-pyrrolidine acetate is hydrolyzed to give the corresponding racemic acid in the presence of sodium hydroxide; said acid is subjected to chemical resolution by reaction with an optically active base, (+)-(R)-(1-phenyl ethyl)-amine, selective crystallization of diastereoisomeric salts thereof and isolation of the desired enantiomeric form; finally, the resultant (−)-(S)-alpha-ethyl-2-oxo-1-pyrrolidineacetic acid is converted into the corresponding amide via activation of the carboxyl residue with ethyl chloroformate.
Several alternative processes for the preparation of levetiracetam have been disclosed in the art.
GB 1,309,692 (UCB S.A.) describes the preparation of several N-substituted lactams, including, inter alia, 2-(2-oxo-pyrrolidino)-butyramide, i.e. the racemic form of levetiracetam, by converting the corresponding ester, obtained by reacting the appropriate pyrrolidin-2-one with an appropriate alkyl haloalkylcarboxylate, with gaseous ammonia in methanol (example 2) or by converting the corresponding acid chloride, obtained by reacting the corresponding acid with thionyl chloride, with gaseous ammonia (example 3).
WO 01/64637 (UCB Farchim) describes the preparation of levetiracetam by asymmetric hydrogenation in the presence of a chiral catalyst of (Z) or (E)-2-(2-oxotetrahydro-1H-1-pyrrolyl)-2-butenamide, which in turn is obtained by reacting the corresponding acid with PCl5 to give the corresponding acid chloride, and then with gaseous ammonia.
WO 03/014080 (UCB S.A.) describes a process for the preparation of levetiracetam and analogues thereof comprising the synthesis of the corresponding ester derivative, methyl-(S)-alpha-ethyl-2-oxo-1-pyrrolidine-acetate, and the subsequent ammonolysis reaction in the presence of water.
EP 1,566,376 (REDDYS LAB LTD DR) discloses a process for the preparation of levetiracetam by reacting 4-chlorobutyl chloride with (S)-2-Aminobutyramide hydrochloride, this latter being obtained by first reacting (5)-2-aminobutyric acid hydrochloride with thionyl chloride in methanol to give the corresponding ester hydrochloride, and then reacting the corresponding ester with ammonia in isopropanol.
Several other patents and patent applications describe other approaches to the synthesis of levetiracetam, such as, for example, U.S. Pat. Nos. 6,107,492 and 6,124,473 which describe the preparation of levetiracetam by optical resolution of etiracetam by means of preparative high performance liquid chromatography or continuous simulated moving bed chromatographic system, GB 2,225,322, which describes a process for the preparation of levetiracetam by hydrogenolysis of (S)-alpha-[2-(methylthio)-ethyl]-(2-oxo-1-pyrrolidine)-acetamide in the presence of a desulfurizing agent, and WO 2004/069796, which describes a process for preparing levetiracetam which comprises reacting (S)-2-aminobutyrramide hydrochloride and 4-chlorobutyl chloride in a solvent selected from acetonitrile and methyl tertbutyl ether in the presence of a strong base and recovering the crude product.
EXAMPLE 1Invention
Step 1
(−)-(S)-alpha-ethyl-2-oxo-1-pyrrolidine acetic acid (150 g, 0.87 mol) was dissolved in methanol (235 g, 300 ml) at 45° C. and thionyl chloride (56 g, 0.47 mol) was added dropwise over 30 min.
The reaction mixture was stirred at 45° C. for additional 15-30 min until complete conversion of (−)-(S)-alpha-ethyl-2-oxo-1-pyrrolidine acetic acid was observed via HPLC (unreacted (−)-(S)-alpha-ethyl-2-oxo-1-pyrrolidine acetic acid ≦2%, by HPLC % area).
At reaction completed, the volatiles were distilled off at moderate temperature and reduced pressure (35°-40° C., 150-200 mbar) until 10% of the whole volume was eliminated, then the mixture was reintegrated with fresh methanol up to initial volume.
After that, the reaction mixture was neutralized by bubbling ammonia gas at 20° C. up to a pH value equal to about 5, and stirred at 20° C. for 1 h. A limited amount of salts (about 44 g) precipitated and was filtered off. The resulting methanol solution was directly transferred to the autoclave.
Step 2
The reaction mixture was pressurized up to about 3 bar with ammonia gas at 20° C., and stirred until complete conversion to (−)-(S)-alpha-ethyl-2-oxo-1-pyrrolidineacetamide was observed via HPLC.
Then, once the reaction mixture was taken out of the autoclave, the residual salts formed (about 20 grams) were filtered off and the methanol solution was distilled up to a minimum volume at moderate temperature and reduced pressure (35°-40° C., 150-200 mbar).
Acetone (115 ml) was added and the mixture was distilled again at moderate temperature and reduced pressure (35°-40° C., 150-200 mbar) to minimum volume. After that acetone (300 ml) was charged over the residue and the mixture was heated and refluxed for 30 minutes. Finally, the solution was cooled down slowly to 0° C. and crude levetiracetam was isolated by filtration.
Crude levetiracetam (molar yield 73.1%, (R)-enantiomer: 1.171%) was then submitted to a final purification process in one step to give pure levetiracetam.
Acetone (750 ml) was charged over crude levetiracetam and the mixture was again stirred and heated to reflux. Once refluxed for about 30 minutes the hot mixture was filtered to remove residual salts and cooled slowly to 0° C.
Pure levetiracetam ((R)-enantiomer: 0.01%) was obtained by filtration and drying under vacuum at 40° C. Overall molar yield was 60.0% by mole of the starting amount of (−)-(S)-alpha-ethyl-2-oxo-1-pyrrolidine acetic acid (ponderal yield 78.4% by weight).
EXAMPLE 2
Example 1 was repeated, but the neutralization step with ammonia at the end of step 1 was omitted. At the end of step 2, crude levetiracetam was isolated (molar yield 73.1%, (R)-enantiomer: 2.21%). After purification step, pure levetiracetam (molar yield 64.4%, (R)-enantiomer: 0.58%) was obtained.
EXAMPLE 3Comparison
Step 1 of Example 1 was repeated using an excess of thionyl chloride (114 g, 0.96 mol) with respect to (−)-(S)-alpha-ethyl-2-oxo-1-pyrrolidine acetic acid. Further, when the complete conversion of (−)-(S)-alpha-ethyl-2-oxo-1-pyrrolidine acetic acid was observed, the reaction mixture was distilled off at moderate temperature and reduced pressure (35°-40° C., 150-200 mbar) until dryness. Decomposition of about 13% by weight of the intermediate product to starting product (−)-(S)-alpha-ethyl-2-oxo-1-pyrrolidine acetic acid was observed.
EXAMPLE 4Effect of Activating Agent Amount
Example 1 was repeated using different amount of thionyl chloride as reported in the following Table 1. The amount of thionyl chloride is expressed in terms of equivalent with respect to (−)-(S)-alpha-ethyl-2-oxo-1-pyrrolidine acetic acid.
| TABLE 1 | ||||
| Conversion | Unreacted (% | Converted (% | ||
| Sample | SOCl2 | Time (hours) | w/w) | w/w) |
| 1 | 1.10 | 1 | 0.1 | 99.9 |
| 2 | 0.78 | 0.5 | 0.4 | 99.7 |
| 3 | 0.60 | 1 | 1.1 | 99.3 |
| 4 | 0.54 | 0.5 | 1.1 | 99.3 |
| 5 | 0.29 | 2 | 2.2 | 98.4 |
| 6 | 0.09 | 5 | 4.9 | 96.7 |
| 7 | 0.05 | 24 | 1.7 | 99.0 |
The data of Table 1 clearly show that the use of a substoichiometric amount of thionyl chloride (samples 2 to 5) does not substantially affect the conversion time and conversion yield of (−)-(S)-alpha-ethyl-2-oxo-1-pyrrolidine acetic acid. On the contrary, the use of catalytic amount of thionyl chloride (samples 6 and 7) substantially increases the conversion time and/or the conversion yield.
………………………..

Compound (I) can also be condensed with 4-chlorobutyryl chloride (IV) either directly in the presence of tetrabutylammonium bromide (TBAB) in dichloromethane, followed by in situ treatment with potassium hydroxide, or via the isolation of intermediate (S)-N-[1-(carbamol)propyl]-4-chlorobutyramide (V).
……………..

An alternative procedure involves hydrolysis of racemic ethyl 2-(2-oxopyrrolidin-1-yl)burytate (VI) with sodium hydroxide to produce racemic 2-(2-oxopyrrolidin-1-yl)butyric acid (VII), which is resolved by fractional crystallization with (R)-(+)-alpha-methylbenzylamine in benzene, followed by acid-base treatment to give (S)-2-(2-oxopyrrolidin-1-yl)butyric acid (VIII). Compound (VIII) is finally treated with ethyl chloroformiate and ammonia in dichloromethane
………………………….
US Patent 8,338,621
J. Surtees and co-inventors disclose alternative processes for making active pharmaceutical ingredients (APIs) that are used to treat epilepsy and seizures. One compound that can be prepared by their processes is the established drug levetiracetam (1, Figure 1), marketed under the trade name Keppra. Because 1 is now off-patent, there is obvious interest in new drugs.

The inventors also claim that seletracetam (2) and brivaracetam (3) (Figure 2) can be prepared by their processes. These drugs are apparently much more active than 1.
All of the drugs are used as single isomers, so a stereoselective synthesis is desirable. The inventors describe two routes for preparing the molecules; the first, shown in Figure 1, is the synthesis of 1 by the reaction between pyrrolidone (4) and chiral bromo amide 5 in the presence of a base. GC analysis showed that the conversion is 40.3% and that the product contains 51% of the (S)-enantiomer and 49% of the (R)-isomer. No details of their separation are given, although the use of chiral HPLC is discussed.
The same reaction is used to prepare derivative 6 of 1. Compound 7 is prepared from the corresponding hydroxy ester and then condensed with 4 to give 6. Chiral HPLC showed that the product is a mixture of 89.3% (S)-enantiomer 6and 10.7% of its (R)-isomer.
The inventors do not describe the detailed preparation of 2, but they report that acid 8 is prepared in 41% yield from pyrrolidone 9 and acid 10 in the presence of NaH (Figure 2). Ammonolysis of 8 produces 2; no reaction details are provided.

In a reaction similar to the preparation of 8, acid 11 is prepared from 10 and pyrrolidone 12. The product is isolated in 77% yield and can be converted to 3by ammonolysis. Again, no details are provided for this reaction.
The second route for preparing the substituted pyrrolidones does not start with simple pyrrolidones and is the subject of additional claims. The route involves a cyclization reaction, shown in Figure 3. The preparation of enantiomer 13 begins with the reaction of racemic salt 14 and optically pure bromo ester 15. This step produces intermediate 16, isolated as a yellow oil. The crude material is treated with 2-hydroxypyridine (2-HP) to cyclize it to 17. This ester is hydrolyzed to give acid 18. Conversion to 13 is carried out by adding ClCO2Et, followed by reaction with liquid NH3 in the presence of K2CO3. The overall yield of 13 is 32%.

This route is also used to prepare levetiracetam (1) by treating 5 with the HCl salt of amino ester 19 to give 20, recovered as its HCl salt in 49% yield. The salt is basified with Et3N and treated with 2-HP to cyclize it to 1, initially isolated as an oil. GC analysis showed 100% conversion, and chiral HPLC showed that the product contains 98.6% (S)-isomer and 1.4% (R)-isomer.
The inventors also prepared 1 and its (R)-enantiomer 21 by using a similar reaction scheme with alternative substrates to 5. Figure 4 outlines the route, which starts from protected hydroxy amide 22 and amino ester 23. When the reaction is carried out in the presence of Cs2CO3, the product is (R)-enantiomer24, which is used without purification to prepare 21 by treating it with 2-HP. Chiral HPLC showed that the product is 94% (R) and 6% (S).

When the reaction between 22 and 23 is run with K2CO3, the product is (S)-enantiomer 25. This is used to prepare 1, but the product contains only 79% (S)-isomer.
The inventors do not comment on the apparent stereoselectivity of the carbonate salts in the reaction of 22 with 23. This is an intriguing finding and worthy of investigation. (UCB S.A. [Brussels]. US Patent 8,338,621,
…………………
Production of Levetiracetam
(1)H-MET-NH2 can be used to manufacture Levetiracetam. The detail is as follows:

(2)A reaction flask was added 500ml of methanol and deionized water 33ml, cooled to 0 ° C. Then add with stirring 50.0g (0.27mol), pass ammonia and dissolve to saturation, and seale reaction flask 0 to 5 º C reaction was stirred 96h TLC tracking,eluent, ethyl acetate / acetone (3:1) product Rf = 0.28, raw material Rf = 0.6]. feedstock point disappears, and the end of the reaction. Finanly, it was distilled under reduced pressure to obtain a yellow solid levetiracetam crude product 41.5g and the yield is 90.2%.

SYNTHESIS
SYN 1
UCB PHARMA, S.A. Patent: WO2007/65634 A1, 2007 ; Location in patent: Page/Page column 16-17 ;
941289-97-0![]()
LEVETIRACETAM
SYN 2
TEVA PHARMACEUTICAL INDUSTRIES LTD.; TEVA PHARMACEUTICALS USA, INC. Patent: WO2004/69796 A2, 2004 ; Location in patent: Page 9 ;
 AND
AND  GIVES PDT
GIVES PDT
SYN 3
 GIVES PDT
GIVES PDT
ZACH SYSTEM S.P.A. Patent: US2011/65932 A1, 2011 ; Location in patent: Page/Page column 3 ;
SYN 4

ZaCh System S.p.A. Patent: EP2147911 A1, 2010 ; Location in patent: Page/Page column 5 ;
SYN 5
 AND
AND  GIVES PDT
GIVES PDT
U C B Societe Anonyme Patent: US4696943 A1, 1987 ;
SYN 6
 AND
AND
UCB, S.A. Patent: WO2005/28435 A1, 2005 ; Location in patent: Page/Page column 10 ;
SYN 7

Tetrahedron Letters, , vol. 47, # 38 p. 6813 – 6815
SYN 8

WO2004/69796 A2, ; Page 11 ;
SEE ALSO
Tetrahedron Asymmetry, , vol. 16, # 22 p. 3739 – 3745
WO2008/77035 A2, ; Page/Page column 15 ;
EP1806339 A1, ; Page/Page column 22 ;
……………

Green Chemistry Letters and Reviews Vol. 3, No. 3, September 2010, 225230
http://www.tandfonline.com/doi/pdf/10.1080/17518251003716568
The desired compound 1 was re-crystallized in hot ethyl acetate (2106 ml) at 60degC, subsequently cooled to 25-30degC, filtered and dried at 35-40degC to obtain product in 65% yield (13 g) and 99.9% purity (by chiral HPLC) as a white solid: mp 116degC (lit3c 117degC); Rf: 0.34 [3:7 (EtOAc: Hexane)]; IR (KBr) nmax 3362, 3200, 2991, 2911, 1676, 1491, 1457, and 1383 cm1 ; 1 H NMR (400 MHz, CDCl3) d 0.91 (t, 3H, J7.5 Hz), 1.601.75 (m, 1H), 1.902.09 (m, 3H), 2.382.47 (m, 2H), 3.343.55 (m, 2H), 4.44 (dd, 1H, J6.7, 8.6 Hz) 5.74 (br, 1H s), 6.45 (br, 1H, s).
3 ) Kotkar, S.P.; Sudalai, A. Tetrahed. Lett. 2006, 47, 68136815.
……..

http://www.google.com/patents/WO2008012268A1?cl=en
Levetiracetam, (-)-(S)-alpha-ethyl-2-oxo- 1 -pyrrolidineacetamide, is a drug useful as a protective agent for treating and preventing hypoxic and ischemic type aggressions of the central nervous system. It is the active ingredient of KEPPRA®, tablets and flavored liquid, indicated as adjunctive therapy in the treatment of partial onset seizures in adults and children four years of age and older with epilepsy. Levetiracetam was first described in US 4,837,223 (UCB Societe Anonyme) where it is stated that it has particular therapeutic properties compared to the known racemic form (non proprietary name etiracetam). The S-enantiomer, for example, has a ten times higher protective activity against hypoxia and a four times higher protective activity against cerebral ischemia than the racemic mixture US ‘223 describes a method for the preparation of levetiracetam which comprises reacting (-)-(S)-alpha-ethyl-2-oxo-l-pyrrolidineacetic acid successively with alkylhaloformate and with ammonia. Said acid intermediate is, in turn, obtained from racemic (±)-alpha-ethyl-2-oxo-l -pyrrolidine acetic acid by a classic optical resolution according to known methods. In example 1 of the above US patent, ethyl (±)-alpha-ethyl-2-oxo-l -pyrrolidine acetate is hydrolyzed to give the corresponding racemic acid in the presence of sodium hydroxide; said acid is subjected to chemical resolution by reaction with an optically active base, (+)-(R)-(l -phenyl ethyl)-amine, selective crystallization of diastereoisomeric salts thereof and isolation of the desired enantiomeric form; finally, the resultant (-)-(S)-alpha-ethyl-2-oxo-l-pyrrolidineacetic acid is converted into the corresponding amide via activation of the carboxyl residue with ethyl chloroformate, in accordance with the following reaction scheme:
Several alternative processes for the preparation of levetiracetam have been disclosed in the art. WO 03/014080 (UCB S.A.) describes an improved process for the preparation of levetiracetam and analogues thereof comprising the ammonolysis reaction of the corresponding ester derivatives in the presence of water.
US 6,107,492 (Daicel Chem; UCB) and US 6,124,473 (UCB) describe the preparation of levetiracetam by optical resolution of etiracetam by means of preparative high performance liquid chromatography or continuous simulated moving bed chromatographic system.
GB 2,225,322 (UCB) describes a process for the preparation of levetiracetam by hydrogenolysis of (S)-alpha-[2-(methylthio)-ethyl]-(2-oxo-l-pyrrolidine)-acetamide in the presence of a desulfurizing agent such as NaBH4/NiC12 6 H2O, nickel Raney W-2 or nickel Raney T- 1.
WO 01/64637 (UCB Farchim) describes the preparation of levetiracetam by asymmetric hydrogenation of (Z) or (E)-2-(2-oxotetrahydro-lH-l-pyrrolyl)-2- butenamide by using a chiral catalyst. EP 162,036 (UCB) describes the preparation of levetiracetam by reacting (S)-2- aminobutanamide with an alkyl 4-halobutyrate or with a 4-halobutyryl halide, and subsequent cyclization of alkyl (S)-4-[[l-(aminocarbonyl)-propyl]-amino-butyrate or of (S)-N-[l-(aminocarbonyl)-propyl]-4-halobutanamide thus obtained. WO 2004/069796 (Teva Pharmaceutical Industries) describes a process for preparing levetiracetam which comprises reacting (S)-2-aminobutyrramide hydrochloride and 4-chlorobutyl chloride in a solvent selected from acetonitrile and methyl tertbutyl ether in the presence of a strong base and recovering the crude product. US 2005/0182262 (Dr. Reddy’s Laboratories) describes the preparation of (S)-2- aminobutyrramide hydrochloride, intermediate useful for the manufacture of levetiracetam via reaction with 4-chlorobutyl chloride.
WO 2004/076416 (Farma Lepori S.A.) describes a process to levetiracetam by means of deaminomethylation of a sufficiently pure enantiomer S-intermediate of formula
or a salt thereof.
In accordance with US ‘223, (-)-(S)-alpha-ethyl-2-oxo-l-pyrrolidineacetamide can not be obtained directly from the racemic mixture by separating the desired enantiomer.
Thus, as underlined above, in US ‘223 the resolution step is carried out on the intermediate (±)-alpha-ethyl-2-oxo-l-pyrrolidineacetic acid.
Said procedure has an intrinsic drawback due to separation of the S-enantiomer from the corresponding racemic mixture by classic optical resolution which, necessarily, leads to a loss of 50% of the acid substrate used.
Example 6 (-)-(S)-alpha-ethyl-2-oxo-l-pyrrolidineacetamide (levetiracetam).
In a 25 ml flask equipped with thermometer, mechanical stirring and bubble condenser, 3.344 g of (-)-(S)-alpha-ethyl-2-oxo-l-pyrrolidineacetic acid (19.58 mmol, e.e.= 95.0%), 0.11 ml of concentrated sulfuric acid (95.6% m/m, 1.97 mmol) and 17 ml of methanol were charged under nitrogen atmosphere at room temperature. Reaction mixture was heated up to 65°C temperature by oil bath and maintained at reflux temperature up to complete disappearing of starting material (about 2.5 h; checked by TLC, Rf = 0.58 CH2Cl2:Me0H:Ac0H 80:20: 1/silica gel). Reaction mixture was concentrated under vacuum up to a residue was formed then water (2.0 ml) was added. In a 25 ml flask equipped with magnetic stirring and condenser, 7.5 ml of 30% aqueous ammonia solution was charged and cooled to 00C temperature and, keeping under stirring, the aqueous solution of crude (-)-(S)-alpha-ethyl-2-oxo- 1-pyrrolidineacetic acid methyl ester was charged dropwise. When addition was completed, reaction mixture was thermostabilized at 200C and said conditions were maintained overnight.
At complete conversion (about 10 h) excess of ammonia was eliminated by vacuum evaporator. Reaction mixture was extracted with dichloromethane (2 x 3.5 ml), transferred into a continuous liquid-liquid extractor and then refluxed with 7 ml of dichloromethane for 6 hours. Collected organic phases were concentrated under vacuum up to a residue was formed. 2.666 g of a yellow solid was obtained which was suspended in 15.0 ml of acetone. Reaction mixture was heated up to 600C temperature so that complete dissolution of the solid was reached. Then, mixture was slowly cooled. White solid was isolated by filtration, washed with mother liquors and then with 3 ml of cold acetone and, finally, dried in oven under vacuum at 400C temperature for 4 hours to give 2.259 g of levetiracetam (13.274 mmol, 67.8% yield, e.e. 99.9%).
Example 7
(-)-(S)-alpha-ethyl-2-oxo-l-pyrrolidineacetic acid methyl ester. In a 250 ml reactor equipped with mechanical stirring, thermometer and condenser, 2.5 g of (-)-(S)-alpha-ethyl-2-oxo-l-pyrrolidineacet-N-(+)-(R)-(l-phenylethyl)-amide (9.112 mmol, d.e.= 99.3%), 24.85 g (6 eq.) of p-toluensulfonic acid supported by polymeric matrix (30.00-60.00 mesh, 2.2 mmol/g) and 75 ml of toluene were charged. To the reaction mixture was added 0.660 ml (36.64 mmol) of water under stirring and mixture was heated up to reflux temperature. Reaction was monitored by HPLC and at complete conversion of starting material (about 6 h), mixture was cooled to 600C temperature and 75 ml of methanol added. Reaction mixture was maintained at that temperature for 3 h up to complete formation of (-)-(S)-alpha- ethyl-2-oxo-l-pyrrolidineacetic acid methyl ester. Reaction mixture was permitted to cool and then it was filtered on gootch in order to separate the product from the resin. Resin was washed with methanol (2 x 75 ml) and organic phases were collected to give 365.1 g of a 0.462% organic solution of (-)-(S)-alpha-ethyl-2-oxo-l- pyrrolidineacetic acid methyl ester (1.69 g, 9.110 mmol, 100.0% yield) which was used in the following synthetic step. In order to recover (+)-(R)-(l-phenylethyl)-amine, resin was treated with 100 ml of 30% aqueous ammonia solution, 100 ml of methanol, 100 ml of 30% aqueous soda and again with 100 ml of methanol. Resin was then regenerated by washing with HCl 6 M (100 ml) and water up to neuter pH of the eluted phase. Finally, resin was washed with 100 ml of methanol and dried in oven at 500C temperature under vacuum overnight.
Example 8
(-)-(S)-alpha-ethyl-2-oxo-l-pyrrolidineacetamide (levetiracetam) (alternative 1). 365.1 g of the solution of (-)-(S)-alpha-ethyl-2-oxo-l-pyrrolidineacetic acid methyl ester (0.462%, 1.69 g, 9.110 mmol) obtained in Example 7 was charged in a flask and concentrated up to a residue was formed. 2.482 g of a brown oil was obtained. Residue was charged in a 10 ml flask equipped with magnetic stirring and condenser. Reaction mixture was cooled to 00C temperature and, keeping under stirring, 0.8 ml of water and 3.2 ml of 30% aqueous ammonia solution were charged dropwise in about 10 minutes. When addition was completed, reaction mixture was thermostabilized at 200C and said conditions were maintained overnight.
At complete conversion (about 14 h) excess of ammonia was eliminated by vacuum evaporator. Reaction mixture was then extracted with dichloromethane (10 x 5 ml). Collected organic phases were dried on Na2SO4, and concentrated under vacuum up to a residue was formed. 1.999 g of a yellow solid was obtained which was suspended in 5 ml of acetone. Reaction mixture was heated up to 600C temperature so that complete dissolution of the solid was reached. Then, mixture was slowly cooled. White solid was isolated by filtration, washed with mother liquors and then with 1 ml of cold acetone and, finally, dried in oven under vacuum at 25°C temperature for 1 night to give 0.965 g of levetiracetam (5.669 mmol, 62.2% yield, e.e. 94.2%). Example 9
(-)-(S)-alpha-ethyl-2-oxo-l-pyrrolidineacetamide (levetiracetam) (alternative 2). In a 50 ml reactor equipped with mechanical stirring, thermometer and condenser, 0.275 g of (-)-(S)-alpha-ethyl-2-oxo-l-pyrrolidineacet-N-(+)-(R)-(l-phenylethyl)- amide (1.0 mmol, d.e.= 99.3%), 10.0 g of ethyl-thiophenyl-sulfonic acid supported on silica (0.6 mmol/g, supplied by Phosphonics ®) and 15 ml of toluene were charged. To the reaction mixture was added 0.075 ml (4.0 mmol) of water under stirring and mixture was heated up to reflux temperature. Reaction is monitored by HPLC and at complete conversion of starting material (about 5 h), reaction mixture was cooled to 600C temperature and 10 ml of methanol added. Reaction mixture was maintained at that temperature for 3 h up to complete formation of (-)-(S)-alpha-ethyl-2-oxo-l- pyrrolidineacetic acid methyl ester. Reaction mixture was permitted to cool and then worked up according to the procedure described in example 7. 57.9 g of a 0.280% organic solution of (-)-(S)-alpha-ethyl-2-oxo-l-pyrrolidineacetic acid methyl ester (0.162 g, 0.875 mmol, 87.5% yield) was thus obtained. Such solution was charged in a flask and concentrated up to a residue was formed. 0.486 g of a brown oil was obtained. Residue was charged in a 5 ml flask equipped with magnetic stirring and condenser. Reaction mixture was cooled to 00C temperature and, keeping under stirring, 1.5 ml of 30% aqueous ammonia solution were charged dropwise. When addition was completed, reaction mixture was thermostabilized at 200C and said conditions were maintained overnight.
At complete conversion (about 15 h) excess of ammonia was eliminated by vacuum evaporator. Reaction mixture was then extracted with dichloromethane as described in example 8. Recrystallization of the crude product from refluxing acetone afforded 0.076 g of levetiracetam (0.447 mmol, 44.6% yield compared to the starting amide, e.e. 99.9%).
……………

PAPER FROM HINDAWI
Journal of Chemistry Volume 2013 (2013), Article ID 176512, 5 pages http://dx.doi.org/10.1155/2013/176512
Enantioselective Synthesis of Antiepileptic Agent, (−)-Levetiracetam, through Evans Asymmetric Strategy
1Department of Research and Development, Inogent Laboratories Private Limited, 28A, IDA Nacharam, Hyderabad 500 076, India
2Centre for Pharmaceutical Sciences, Institute of Science and Technology, Jawaharlal Nehru Technological University, Kukatpally, Hyderabad 500 072, India
3R&D Centre, Orchid Chemicals and Pharmaceuticals Ltd., 476/14, Sholinganallur, Chennai 600 119,
http://www.hindawi.com/journals/jchem/2013/176512/
A practical and efficient enantioselective synthesis of antiepileptic drug, (−)-Levetiracetam, has been described in five steps (33.0% overall yield) and high optical purity (99.0% ee), using Evans asymmetric strategy for -alkylation of carbonyl functionality as the key step. The simplicity of the experimental procedures and high stereochemical outcome make this method synthetically attractive for preparing the target compound on multigram scales.

white solid. Mp: 113–114°C.
S ROT= −95.0 [c = l.0, acetone].
1H NMR , 400 MHz):
δ 6.50 (br s, 1H),
5.70 (br s, 1H),
4.50 (t, J = 8.7, 6.8 Hz, 1H),
3.48 (m, 2H), 2.50 (m, 2H),
1.98–2.20 (m, 3H),
1.70 (m, 1H),
0.98 (t, J = 7.7 Hz, 3H) ppm; CH2–CH3
13C NMR , 75 MHz): δ175.9, 172.7, 55.9, 43.7, 31.0, 21.2, 18.0, 10.4 ppm;
IR : 3200, 1731, 1620 cm−1;
ESI-MS: m/z 171.0 [M++1].
Anal. calcd. for C8H14N2O2: C, 56.45; H, 8.29; N, 16.46; O, 18.80. Found: C, 56.76; H, 8.52; N, 16.87; O, 19.26.
Chiral HPLC purity 99% ee. The enantiomeric excess was determined by HPLC analysis in comparison with authentic racemic material and HPLC conditions: Chiral OD-H column; hexane: i-PrOH (90 : 10 v/v); flow rate 1.0 mL/min; UV −210 nm; column temperature 25°C; CHIRAL HPLC purity: = 14.4 min (S)-isomer (major enantiomer) and 9.3 min (R)-isomer (minor enantiomer).
…………………

Indian Journal of Chemistry -Section B (IJC-B) >
IJC-B Vol.53B [2014] >
IJC-B Vol.53B(09) [September 2014] >
http://nopr.niscair.res.in/handle/123456789/29370

1H nmr predict

 13 C NMR PREDICT
13 C NMR PREDICT
…

| US4837223 | Mar 12, 1987 | Jun 6, 1989 | Ucb Societe Anonyme | (S)-alpha-ethyl-2-oxo-1-pyrrolidineacetamide compositions |
| US6107492 | May 7, 1999 | Aug 22, 2000 | Ucb, S.A. | By optical resolution of a racemic mixture of alpha-ethyl-2-oxo-1-pyrrolidine acetamide by chromatography using silica gel supporting amylose tris(3,5-dimethylphenylcarbamate) as a packing material |
| US6124473 | May 7, 1999 | Sep 26, 2000 | Ucb, S.A. | Process for preparing (s)- and (R)-α-ethyl-2-oxo-1-pyrrolidineacetamide |
| US7531673 * | Feb 16, 2005 | May 12, 2009 | Dr. Reddy’s Laboratories Limited | Preparation of amino acid amides |
| US20050182262 * | Feb 16, 2005 | Aug 18, 2005 | Acharyulu Palle V.R. | Reacting an amino acid or acid salt with a halogenating agent (thionyl chloride, phosphorous pentachloride or oxalyl chloride) , to form an intermediate, reacting the intermediate with ammonia; amidation; chemical intermediate to form Levetiracetam |
| EP1566376A1 | Feb 17, 2005 | Aug 24, 2005 | Dr. Reddy’s Laboratories Limited | Preparation of amino acid amides |
| GB1309692A | Title not available | |||
| GB2225322A | Title not available | |||
| WO2001064637A1 | Feb 21, 2001 | Sep 7, 2001 | Edmond Differding | 2-oxo-1-pyrrolidine derivatives, process for preparing them and their uses |
| WO2003014080A2 | Aug 5, 2002 | Feb 20, 2003 | Celal Ates | Oxopyrrolidine compounds, preparation of said compounds and their use in the manufacturing of levetiracetam and analogues |
| WO2004069796A2 | Feb 3, 2004 | Aug 19, 2004 | Ben-Zion Dolitzky | Process for producing levetiracetam |
| WO2006095362A1 * | Jan 20, 2006 | Sep 14, 2006 | Rubamin Ltd | Process for preparing levetiracetam |
| WO2008012268A1 | Jul 20, 2007 | Jan 31, 2008 | Zach System Spa | Process for the preparation of levetiracetam |
……

http://orgspectroscopyint.blogspot.in/2015/03/rs-alpha-ethyl-2-oxo-l-pyrrolidineacet.html
PREPARATION OF KEY INETERMEDIATE
(±)-(R,S)-alpha-ethyl-2- oxo-l-pyrrolidineacet-N-(+)-(R)-(l-phenylethyl)-amide
methyl (±)-(R,S)-alpha-ethyl-2-oxo-l -pyrrolidine acetate with (+)-(R)-(l-phenylethyl)- amine in toluene in the presence of a base such as sodium hydride or methoxide; crystallization- induced dynamic resolution of the resultant (±)-(R,S)-alpha-ethyl-2- oxo-l-pyrrolidineacet-N-(+)-(R)-(l-phenylethyl)-amide
(R)-(+)-1-Phenylethylamine
33978-83-5
1-Pyrrolidineacetic acid, α-ethyl-2-oxo-, methyl ester

1004767-60-5
1-Pyrrolidineacetamide, α-ethyl-2-oxo-N-[(1R)-1-phenylethyl]-
(±)-(R.S)-alpha-ethyl-2-oxo-l-pyrrolidineacet-N-(+)-(R)-(l-phenylethyl)-amide
Example 1
(±)-(R,S)-alpha-ethyl-2-oxo-l-pyrrolidineacet-N-(+)-(R)-(l-phenylethyl)-amide.
In a 100 ml reactor equipped with mechanical stirring, thermometer and bubble condenser, 13.4 g of (±)-(R,S)-alpha-ethyl-2-oxo-l-pyrrolidineacetic acid methyl ester (71.6 mmol), 8.8 g of (+)-(R)-(l-phenylethyl)-amine (72.5 mmol) and 45 ml of tetrahydrofuran were charged. 3.4 g of NaH (60% dispersion in mineral oil, 85.6 mmol) was added in small portions under nitrogen atmosphere. Reaction mixture was maintained at room temperature for about 2 h. Then, it was heated up to 350C and kept under stirring overnight. Reaction was controlled by TLC (Rf = 0.5, AcOEt/silica gel).
At reaction completed, one night at 35°C temperature, reaction mixture was cooled to room temperature and 30 ml of water was slowly charged. It was transferred into a separatory funnel and was diluted with 30 ml of water and 80 ml of dichloromethane. Phases were separated and the aqueous one was washed with 50 ml of dichloromethane. Collected organic phases were washed with an aqueous acid solution, dried on Na2SO4, filtered and concentrated under vacuum. 19.5 g of an oil residue was obtained which slowly solidified. Solid was suspended in 20 ml of a hexane/dichloromethane 9/1 v/v mixture. It was then filtered, washed with 10 ml of the same solvent mixture and dried at 400C to give 12.1 g of the title compound (44.1 mmol, 61.6% yield) as dry solid.
1H NMR (400.13 MHz, CDCl3, 25 0C): δ (ppm, TMS)
7.35-7.19 (1OH, m),
6.49 (2H, br s),
5.09-5.00 (2H, m),
4.41 (IH, dd, J = 8.3, 7.4 Hz),
4.36 (IH, dd, J = 8.6, 7.1 Hz),
3.49 (IH, ddd, J = 9.8, 7.7, 6.6 Hz),
3.41 (IH, ddd, J = 9.8, 7.7, 6.2 Hz),
3.30 (IH, ddd, J = 9.6, 8.3, 5.5 Hz),
3.13 (IH, ddd, 9.7, 8.5, 6.1 Hz), 2.47-2.38 (2H, m), 2.41 (IH, ddd, J = 17.0, 9.6, 6.3 Hz), 2.26 (IH, ddd, 17.0, 9.5, 6.6 Hz), 2.10-1.98 (2H, m), 2.01-1.89 (IH, m), 1.99-1.88 (IH, m), 1.98-1.85 (IH, m), 1.88-1.78 (IH, m), 1.75- 1.62 (IH, m), 1.72-1.59 (IH, m), 1.45 (3H, d, J = 7.1 Hz), 1.44 (3H, d, J = 7.1 Hz), 0.90 (3H, t, J = 7.4 Hz), 0.86 (3H, t, J = 7.4 Hz).
13C NMR (100.62 MHz, CDCl3, 25 0C): δ (ppm, TMS)
176.05 (CO), 176.00 (CO), 169.08 (CO),
168.81 (CO), 143.59 (Cquat),
143.02 (Cquat), 128.66 (2 x CH), 128.55 (2 x CH),
127.33 (CH), 127.19 (CH), 126.05 (2 x CH),
125.80 (2 x CH), 56.98 (CH), 56.61 (CH),
48.90 (CH), 48.84 (CH), 44.08 (CH2),
43.71 (CH2), 31.19 (CH2), 31.07 (CH2), 22.08 (CH3),
22.04 (CH3), 21.21 (CH2), 20.68 (CH2),
18.28 (CH2), 18.08 (CH2), 10.50 (CH3), 10.45 (CH3).
Example 2 (±)-(R.S)-alpha-ethyl-2-oxo-l-pyrrolidineacet-N-(+)-(R)-(l-phenylethyl)-amide (alternative 1).
In a 500 ml reactor equipped with mechanical stirring, thermometer and condenser, 24.2 g of (+)-(R)-(l-phenylethyl)-amine (199.51 mmol) and 40 ml of toluene were charged. By keeping the reaction mixture at 00C temperature under nitrogen atmosphere, 9.5 g of NaH (60% mineral oil suspension, 237.50 mmol) was added in small portions. At the same temperature, 190.0 g of a toluene solution of (±)-(R,S)- alpha-ethyl-2-oxo-l-pyrrolidineacetic acid methyl ester (19.28% equal to 36.63 g, 197.77 mmol) was charged. Reaction mixture was then heated up to 35°C and maintained in that condition till complete disappearing of methyl ester reagent (about 14 h; checked by HPLC).
At reaction completed, reaction mixture was cooled and when room temperature was reached, 100 ml of water was slowly charged. Aqueous phases were separated and extracted with toluene (2 x 75 ml). Collected organic phases were treated with acid water till neuter pH. Solvent was evaporated and residue was suspended in about 100 ml of heptane for about 30 minutes. Product was isolated by filtration and dried in oven at 400C temperature under vacuum overnight to give 45.2 g of the title compound (164.54 mmol, 83.2% yield, d.e. 0.0%) as white dusty solid.
Example 3
(±)-(R,S)-alpha-ethyl-2-oxo-l-pyrrolidineacet-N-(+)-(R)-(l-phenylethyl)-amide (alternative 2).
In a 500 ml reactor equipped with mechanical stirring, thermometer and Dean-Stark distiller, 24.2 g of (+)-(R)-(l-phenylethyl)-amine (199.51 mmol) and 40 ml of toluene were charged. By keeping the reaction mixture at 00C temperature, 42.7 g of sodium methoxide (30% solution in methanol, 237.14 mmol) was added under nitrogen atmosphere. At the same temperature, 190.0 g of a toluene solution of (±)- (R,S)-alpha-ethyl-2-oxo-l-pyrrolidineacetic acid methyl ester (19.28% equal to 36.63 g, 197.77 mmol) was charged. Reaction mixture was then heated up to 65- 700C and maintained in that condition till complete disappearing of methyl ester reagent (about 4 h; checked by HPLC). After a work-up carried out according to the procedure described in example 2, 40.2 g of the title compound (146.53 mmol, 74.1% yield, d.e. 0.0%) as white dusty solid was obtained.
…….

 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO
.....
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO
.....
















Reblogged this on ORGANIC CHEMISTRY SELECT.
Reblogged this on MedCheminSingapore by Sushma Wang.
Reblogged this on MED.CHEM in BURMA.
Reblogged this on Organic Reactions in Medchem.
Heya i’m for the first time here. I came across this board and I to find It truly helpful
& it helped me out a lot. I hope to give one thing back and aid
others such as you helped me.