
 ondansetron
ondansetron
Ondansetron hydrochloride dihydrate, cas 99614-01-4, GG-032, SN-307, GR-C505/75,
Ondansetron hydrochloride dihydrate is a serotonin-3 (5-HT3) receptor antagonist. J Org Chem1980, 45, (15): 2938 Heterocycles1997, 45, (10): 2041 EP 0595111 WO 0172716, Ondansetron (INN) (/ɒnˈdænsɛtrɒn/; developed and first marketed by GlaxoSmithKline as Zofran) is a serotonin 5-HT3 receptor antagonist used mainly as an antiemetic (to treat nausea and vomiting), often following chemotherapy. It affects both peripheral and central nerves. Ondansetron reduces the activity of the vagus nerve, which deactivates the vomiting center in the medulla oblongata, and also blocks serotonin receptors in thechemoreceptor trigger zone. It has little effect on vomiting caused by motion sickness, and does not have any effect on dopamine receptors ormuscarinic receptors. Although an effective anti-emetic agent, the high cost of brand-name ondansetron initially limited its use to controlling postoperative nausea and vomiting (PONV) and chemotherapy-induced nausea and vomiting (CINV). The active ingredient in ZOFRAN Tablets and ZOFRAN Oral Solution is ondansetron hydrochloride (HC1) as the dihydrate, the racemic form of ondansetron and a selective blocking agent of the serotonin 5-HT3 receptor type. Chemically it is (±) 1, 2, 3, 9-tetrahydro-9-methyl-3-[(2-methyl-lH-imidazol-l-yl)methyl]-4H-carbazol-4-one, monohydrochloride, dihydrate. It has the following structural formula:
 |
The empirical formula is C18H19N3O•HCl•2H2O, representing a molecular weight of 365.9. Ondansetron HC1 dihydrate is a white to off-white powder that is soluble in water and normal saline. The active ingredient in ZOFRAN ODT Orally Disintegrating Tablets is ondansetron base, the racemic form of ondansetron, and a selective blocking agent of the serotonin 5-HT3 receptor type. Chemically it is (+) 1, 2, 3, 9-tetrahydro-9-methyl-3-[(2-methyl-lH-imidazol-l-yl)methyl]-4H-carbazol-4-one. It has the following structural formula:
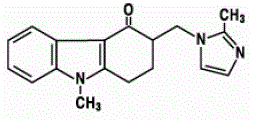 |
The empirical formula is C18H19N3O representing a molecular weight of 293.4. The 5-HT3 receptor antagonists are the primary drugs used to treat and prevent chemotherapy-induced nausea and vomiting (CINV). A common use case is to give them intravenously about 30 minutes before commencement of a chemotherapy treatment.
Ondansetron is used off-label to treat morning sickness and hyperemesis gravidarum of pregnancy. A cohort study of over 600,000 pregnancies in Denmark found that ondansetron administration during pregnancy is not associated with a significantly increased risk of spontaneous abortion,stillbirth, major birth defect, preterm birth, low birth weight, or small for gestational age. However, in practice, ondansetron is typically used after trials of other drugs have failed.
Ondansetron is one of several anti-emetic agents used during the vomiting phase of cyclic vomiting syndrome. Trials in emergency department (ED) settings support the use of ondansetron to reduce vomiting associated with gastroenteritis and dehydration.A retrospective review found that it was used commonly for this purpose, being administered in over 58% of cases. Its use reduced hospital admissions, but was also associated with higher rates of return visits to the ED. Furthermore, patients who had initially received ondansetron were more likely to be admitted on the return visit than patients who had not received the drug. However, this effect may simply be due to the agent being used more frequently in patients who present with more severe illness. Its use was not found to mask serious diagnoses.
Ondansetron was developed around 1984 by scientists working at Glaxo’s laboratories in London. It is in both the imidazole and carbazole families of heterocyclic compounds. After several attempts the company successfully filed for U.S. patent protection for the drug in 1986 and was granted in June 1988 while a use patent was granted in June 1988. A divisional use patent was granted on November 26, 1996. Ondansetron was granted FDA approval as Zofran in January 1991. Glaxo did pediatric research on Zofran’s uses, and gained a patent extension as a result, extending U.S. exclusivity until December 24, 2006. The FDA subsequently approved the first generic versions in December 2006, with marketing approval granted to Teva Pharmaceuticals USA and SICOR Pharmaceuticals.
Ondansetron is marketed by GlaxoSmithKline (GSK) under the trade name Zofran. Other manufacturers include Pfizer Injectables (Ondanzetron), Opsonin Pharma Bangladesh (Anset), Strativa Pharmaceuticals (Zuplenz), Indswift Ltd. (Ondisolv), Cipla Ltd. (Emeset), Gedeon Richter Ltd. (Emetron), Korea United Pharmaceuticals (Emodan), Zentiva a.s. (Ondemet), Strides Arcolab (Setronax), Emistat (Unimed and Unihealth Bangladesh Ltd.)Glenmark Generics Ltd. (India) (Ondansetron) and Novell Pharmaceutical Laboratories (Ondavell). On May 29, 2006, Baxter Healthcare received tentative approval to market its own label of Ondansetron Injection, USP, 8 mg/50 mL and 32 mg/50 mL iso-osmotic sodium chloride solution, beginning upon expiration of GSK’s patent later that year.
In 1997, ondansetron was the subject of a meta-analysis case-study published in the British Medical Journal. Researchers examined 84 trials, with 11,980 patients receiving ondansetron, published between 1991 and September 1996. Intravenous ondansetron 4 mg versus placebo was investigated in 16 reports and three further reports which had been duplicated six times. The number needed to treat (NNT) to prevent vomiting within 24 hours was 9.5, with 95% confidence interval 6.9 to 15, in the 16 non-duplicated reports. In the three duplicated reports, the NNT was significantly lower at 3.9 (3.3 to 4.8) with P<0.00001. When all 25 reports were combined the apparent number needed to treat improved to 4.9 (4.4 to 5.6). Inclusion of duplicate reports led to a 23% overestimation of ondansetron’s antiemetic efficacy. In addition, the authors found that the covert duplication of reports on ondansetron was not easy to detect, because of lack of cross-referencing between papers, and that reports containing duplicate findings were cited in eight reviews of the drug. Their analysis was a subject of an editorial in the Journal of the American Medical Association in 1999. ………………………
patents
AU 8538097; BE 0901576; CH 664152; ES 8609309; ES 8708224; ES 8801247; FR 2561244; GB 2153821; JP 1985214784
……………..
(±) l,2,3,9-Tetrahydro-9-methyl-3-[2-methyl-lh-imidazol-l-yl)methyl]-4h- carbazol-4-one having the molecular structure
is a selective 5-HT3 receptor antagonist. It is known by the generic nameondansetron. Ondansetron reduces nausea in patients undergoing chemotherapy. Grunberg, S.M.; Hesketh, P.J. “Control of Chemotherapy-Induced emesis” N. Engl. J. Med. 1993, 329, 1790-96. Ondansetron is indicated for prevention of nausea and vomiting associated with some cancer chemotherapy, radiotherapy and postoperative nausea and/or vomiting.
Several chemical processes are known from the literature for the synthesis ofondansetron. GB-Pat. 2 153 821 and 2 192 885 describe syntheses starting from carbazolone derivative, and EP-Pat. 595 111 as well as a Hungarian patent application ( P 00-01287 ) give detailed information about some different chemical procedures.
Ondansetron is currently available as an anti-emetic agent, particularly in cancer chemotherapy, and in some other uses such as anti-depressive, anti- migraine and anti-psychotic. It is commonly used in the alleviation of cognitive disorders as in Alzheimer disease, in treatment of rhinitis, psychiatric disorders and for increased vigilance and for control of dependence on narcotics.
U.S. Patent No. 4,695,578, assigned to the Glaxo Group Limited, describes a process of preparing ondansetron and uses thereof. However, ondansetronprepared according to said process contains impurities and by-products such as l,2,3,9-tetrahydro-9-methyl-3-methylene-4H-carbazol-4-one.
The hydrochloride salt of ondansetron is generally safe for oral administration to a patient without causing irritation or other adverse effect. The hydrochloride salt is marketed in tablet form and in oral solution form under the brand name Zofran®. The tablet’s active ingredient is a dihydrate of ondansetronhydrochloride containing two molecules of bound water in ondansetronhydrochloride’ s crystal lattice. The present invention relates to the solid state physical properties of ondansetron hydrochloride. These properties can be influenced by controlling the conditions under which the hydrochloride salt is obtained in solid form. Solid state physical properties include, for example, the flowability of the milled solid. Flowability affects the ease with which the material is handled during processing into a pharmaceutical product. When particles of the powdered compound do not flow past each other easily, a formulation specialist must take that fact into account in developing a tablet or capsule formulation, which may necessitate the use of glidants such as colloidal silicon dioxide, talc, starch or tribasic calcium phosphate.
These important physical characteristics are influenced by the conformation and orientation of molecules in the unit cell, which defines a particular polymorphic form of a substance. Llacer and coworkers have postulated that different spectroscopic characteristics of samples of ondansetron free base prepared differently could be attributable to two different configurations about the methylene bridge between the 1, 2, 3, 9-tetrahydrocarbazol-4-one ring and the imidazole ring. Llacer, J.M.; Gallardo, V.; Parera, A. Ruiz, M.A. InternJ.Pharm., 177, 1999, 221-229.
(±)1,2,3,9-Tetrahydro-9-methyl-3-[2-methyl-1h-imidazol-1-yl)methyl]-4h-carbazol-4-one having the molecular structure
is a selective 5-HT3 receptor antagonist. It is known by the generic nameondansetron. Ondansetron reduces nausea in patients undergoing chemotherapy. Grunberg, S. M.; Hesketh, P. J. “Control of Chemotherapy-Induced emesis” N. Engl. J. Med. 1993, 329, 1790-96. Ondansetron is indicated for prevention of nausea and vomiting associated with some cancer chemotherapy, radiotherapy and postoperative nausea and/or vomiting.
The hydrochloride salt of ondansetron is generally safe for oral administration to a patient without causing irritation or other adverse effect. The hydrochloride salt is marketed in tablet form and in oral solution form under the brand name Zofran®. The tablet’s active ingredient is a dihydrate of ondansetronhydrochloride containing two molecules of bound water in ondansetronhydrochloride’s crystal lattice.
The present invention relates to the solid state physical properties ofondansetron hydrochloride. These properties can be influenced by controlling the conditions under which the hydrochloride salt is obtained in solid form. Solid state physical properties include, for example, the flowability of the milled solid. Flowability affects the ease with which the material is handled during processing into a pharmaceutical product. When particles of the powdered compound do not flow past each other easily, a formulation specialist must take that fact into account in developing a tablet or capsule formulation, which may necessitate the use of glidants such as colloidal silicon dioxide, talc, starch or tribasic calcium phosphate.
Another important solid state property of a pharmaceutical compound is its rate of dissolution in aqueous fluid. The rate of dissolution of an active ingredient in a patient’s stomach fluid can have therapeutic consequences since it imposes an upper limit on the rate at which an orally-administered active ingredient can reach the patient’s bloodstream. The rate of dissolution is also a consideration in formulating syrups, elixirs and other liquid medicaments. The solid state form of a compound may also affect its behavior on compaction and its storage stability.
These important physical characteristics are influenced by the conformation and orientation of molecules in the unit cell, which defines a particular polymorphic form of a substance. Llacer and coworkers have postulated that different spectroscopic characteristics of samples ofondansetron free base prepared differently could be attributable to two different configurations about the methylene bridge between the 1,2,3,9-tetrahydrocarbazol-4-one ring and the imidazole ring. Llacer, J. M.; Gallardo, V.; Parera, A. Ruiz, M. A. Intern.J.Pharm., 177, 1999, 221-229.
A crystalline polymorphic form of a compound may exhibit different thermal behavior from amorphous material or another polymorphic form. Thermal behavior is measured in the laboratory by such techniques as capillary melting point, thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) and can be used to distinguish some polymorphic forms from others. A particular polymorphic form may also give rise to distinct spectroscopic properties that may be detectable by powder X-ray crystallography, solid state 13C NMR spectrometry and infrared spectrometry. There is a wide variety of techniques that have the potential of producing different crystalline forms of a compound. Examples include crystallization, crystal digestion, sublimation and thermal treatment.
U.S. Pat. No. 4,695,578, Example 1a, discloses a preparation ofondansetron by alkylation of 2-methylimidazole with 2,3,4,9 tetrahydro-N,N,N,9-tetramethyl-4-oxo-1H-carbazole-3-methanaminium iodide. In this example,ondansetron was isolated as its hydrochloride salt by suspending the reaction product in a mixture of absolute ethanol and ethanolic HCl, warming the suspension, filtering to remove impurities and precipitating the hydrochloride salt with dry ether.
In Example 10 of the ‘578 patent, ondansetron free base was converted into a hydrochloride salt dihydrate by dissolving the free base in a mixture of isopropanol and water and treating it with concentrated hydrochloric acid. After filtration at elevated temperature, ondansetron was driven out of solution by adding additional isopropanol and cooling. The dihydrate was obtained as a white crystalline solid by recrystallizing it from a 6:10 mixture of water and isopropanol. Ondansetron hydrochloride dihydrate obtained by following Example 10 of the ‘578 patent is denominated Form A in this disclosure. Powdered samples of Form A produce a powder X-ray diffraction pattern essentially the same as the pattern shown in FIG. 1.
U.S. Pat. No. 5,344,658 describes ondansetron having a particular particle size distribution and the use of such ondansetron in a pharmaceutical composition. The particle size of ondansetron hydrochloride dihydrate obtained by crystallization from a solvent is reduced by desolvating them, e.g. by heating, and then exposing the desolvated crystals to a humid atmosphere. A collection of crystals obtained by this particle size reduction process is said to consist exclusively of crystals of less than 250 micron size and to contain 80% or more crystals of less than 63 microns. Crytals size was determined by air jet seive analysis.
According to the ‘658 patent, ondansetron hydrochloride dehydrate having the same particle size distribution as the rehydrated ondansetron hydrochloride also is provided as part of that invention. Since only one process for dehydratingondansetron hydrochloride is described in the ‘658 patent, a dehydrate is evidently the intermediate compound that is rehydrated in the particle size reduction process.
U.S. Pat. Nos. 4,695,578 and 5,344,658 are incorporated herein by reference.
U.S. Pat. No. 4,695,578 (‘578 patent) discloses a process for preparingondansetron hydrochloride dihydrate having a large particle size (e.g., less than about 60% of the particles are smaller than 250 μm). The ‘578 patent process involves the step of cooling a solution of ondansetron hydrochloride, isopropanol, and water, optionally followed by an additional step of recrystallizing from a mixture of water and isopropanol.
U.S. Pat. No. 5,722,720 (the ‘720 patent) discloses a non-conventional technique for reducing particle size. In particular, the ‘720 patent discloses a multistep process in which ondansetron hydrochloride dihydrate is first dried at elevated temperature and reduced or atmospheric pressure, and is then cooled to ambient temperature. The process requires the heating step to be performed until the ondansetron hydrochloride dihydrate is desolvated, and requires the cooling step to be performed until the ondansetron hydrochloride is rehydrated to form ondansetron hydrochloride dihydrate.
The ‘720 patent process has several disadvantages. First, the ‘720 patent process requires a prolonged time period (i.e., 16-24 hours) for the drying/desolvating step, plus an additional prolonged time period for the cooling/rehydrating step. Second, the ‘720 patent process requires vigorous and carefully controlled drying conditions. For example, when the drying step is performed at 48-52° C., a reduced pressure of 100-200 torr is required. When the drying step is performed at ambient pressure, an elevated temperature of 1 00° C. is required.
An overview of the key routes to the best selling 5-membered ring heterocyclic pharmaceuticals
 Corresponding author email
Corresponding author emailA completely different strategy was used in the synthesis of the serotonin 5-HT3 receptor antagonist ondansetron (119, Zofran). In this synthesis a palladium-catalysed intramolecular Heck-reaction was used to build the tricyclic indole core in a short and concise sequence (Scheme 26) [35,36].
![[1860-5397-7-57-i26]](https://i0.wp.com/beilstein-journals.org/bjoc/content/inline/1860-5397-7-57-i26.png)
Alternatively, a direct Fischer indole synthesis between phenylmethyl hydrazine and a cyclic 1,3-dione derivative could be utilised to prepare the desired fully substituted tricyclic core of ondansetron (Scheme 27) [37].
![[1860-5397-7-57-i27]](https://i0.wp.com/beilstein-journals.org/bjoc/content/inline/1860-5397-7-57-i27.png)
- 35………..Godfrey, N.; Coates, I. H.; Bell, J. A.; Humber, D. C.; Ewan, G. B. Process for Preparing N-Heterocyclic Compounds. U.S. Patent 4,957,609, Sept 18, 1990.
- 36…………Iida, H.; Yuasa, Y.; Kibayashi, C. J. Org. Chem. 1980, 45, 2938–2942. doi:10.1021/jo01303a003
- Oxford, A. W.; Eldred, C. D.; Coates, I. H.; Bell, J. A.; Humber, D. C.; Ewan, G. B. Process for Preparing Tetrahydrocarbazolones. U.S. Patent 4,739,072, April 19, 1988.

 DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO
.....
DRUG APPROVALS BY DR ANTHONY MELVIN CRASTO
.....




